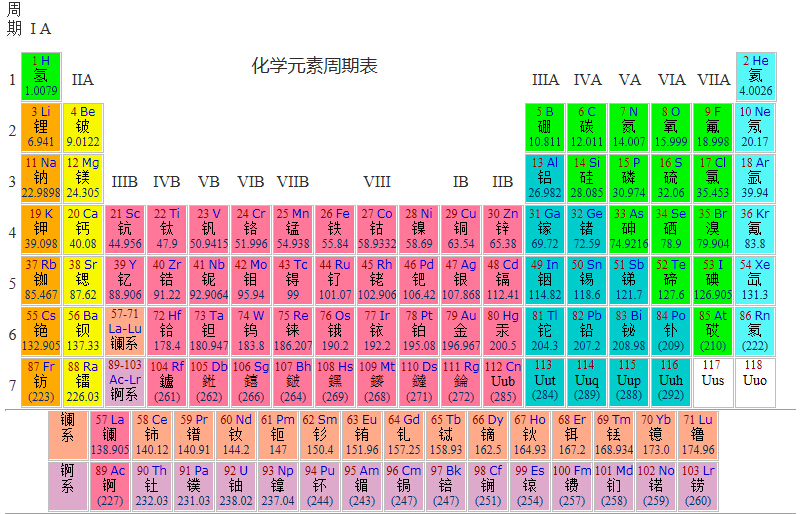
The periodic law of elements in modern chemistry was initiated by Dmitri Mendeleev, a Russian scientist in 1869. He arranged 63 known elements according to atomic weight and in the form of a table, and put elements with similar chemical properties on the same line, which is the prototype of the periodic table of elements. Using the periodic table, Mendeleev successfully predicted the characteristics of elements (gallium, scandium and germanium) that had not been found at that time. In 1913, the British scientist moseler used cathode rays to hit metals to produce X-rays. He found that the larger the atomic order, the higher the frequency of X-rays. Therefore, he believed that the positive charge of the nucleus determined the chemical properties of the elements, and arranged the elements according to the positive charge in the nucleus (i.e. proton number or atomic order). After years of revision, it became a contemporary periodic table.
In the periodic table, elements are arranged in the atomic order of elements, with the smallest ranking first. A row in the table is called a period, and a column is called a family.
Tel
